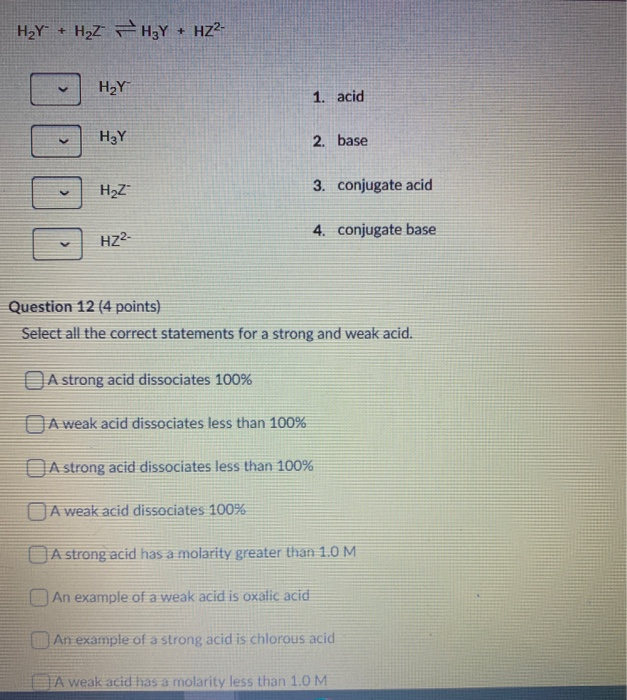
41 h2y acid or base
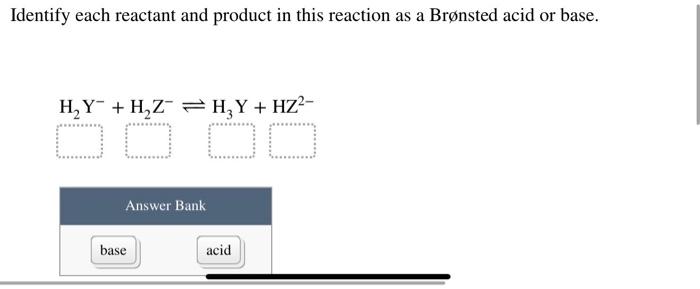
Answered: Identify each reactant and product in… | bartleby Science Chemistry Identify each reactant and product in this reaction as a Brønsted acid or base. H2Y−+H2Z−↽−−⇀H3Y+HZ2− Identify each reactant and product in this reaction as a Brønsted acid or base. H2Y−+H2Z−↽−−⇀H3Y+HZ2− Question Identify each reactant and product in this reaction as a Brønsted acid or base. H2Y−+H2Z−↽−−⇀H3Y+HZ2− Expert Solution Solved Label each reactant and product in this reaction as a | Chegg.com H2Y- + H2Z- <===> H3Y + HZ2- This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Label each reactant and product in this reaction as a Bronsted acid or base. H2Y- + H2Z- <===> H3Y + HZ2- Expert Answer 96% (45 ratings)
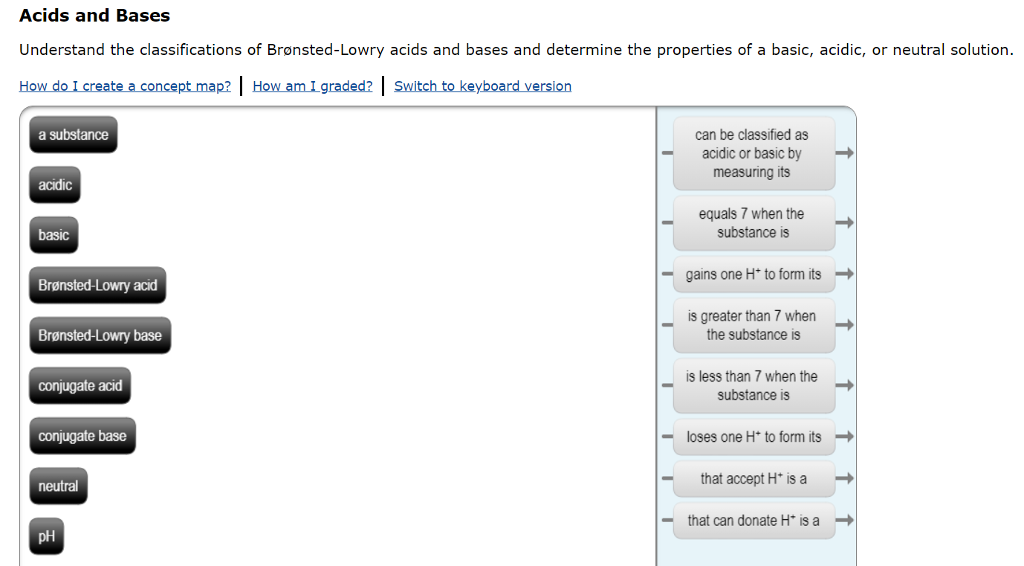
pH Scale: Acids, bases, pH and buffers (article) | Khan Academy The pH scale is often said to range from 0 to 14, and most solutions do fall within this range, although it's possible to get a pH below 0 or above 14. Anything below 7.0 is acidic, and anything above 7.0 is alkaline, or basic. Image modified from " Water: Figure 7 ," by OpenStax College, Biology, CC BY 4.0.
H2y acid or base
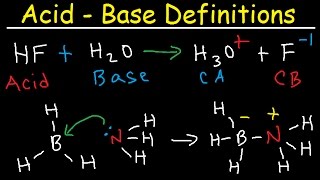
6.1: What is an acid and a base? - Chemistry LibreTexts Arrhenius's definition of acids and bases. The earliest definition of acids and bases is Arrhenius's definition which states that: An acid is a substance that forms hydrogen ions H + when dissolved in water, and; A base is a substance that forms hydroxide ions OH-when dissolved in water.; or example, hydrochloric acid is an acid because it forms H + when it dissolves in water. Inorganic Exam 2 Flashcards | Quizlet HF + CH3CH2OH -> CH3CH2OH+ + F- Identify each reactant and product in this reaction as a Brønsted acid or base. H2Y- + H2Z- -> H3Y + HZ2- Base. Acid. Acid. Base Identify the pair of species that is not a conjugate acid-base pair. H3PO4; HPO2−4 Which reactant is the Lewis acid and which is the Lewis base? FeBr3 is the Lewis Acid Answered: In the reaction between H2Y - and H2Z -… | bartleby In the reaction between H2Y - and H2Z - , if H2Y - acts like a base, and H2Z - acts like an acid, the products of the reaction are: A) H3Y - and HZ- B) HY- and H3Z - C) H3Y and HZ2- D) H2Y and HZ- E) H3Y and HZ Expert Solution Want to see the full answer? Check out a sample QA here See Solution star_border
H2y acid or base. Hydroxy Definition & Meaning - Merriam-Webster The meaning of HYDROXY is being or containing hydroxyl; especially : containing hydroxyl especially in place of hydrogen —usually used in combination. How to use hydroxy in a sentence. Acid and Base Chart — Table of Acids & Bases - Sigma-Aldrich Acid-Base Pairs and Buffer Chemistry A buffer solution contains a weak acid and its conjugate base or a weak base and its conjugate acid. Buffers work by reacting with a base or acid to control the pH of a solution. Download the Acid & Base Chart Acid & Base Chart Image Acid & Base Chart PDF Hydroxy acid | chemical compound | Britannica carboxylic acids. In carboxylic acid: Hydroxy and keto acids. The 2-, 3-, 4-, and 5-hydroxycarboxylic acids all lose water upon heating, although the products are not the same. The 2-hydroxy acids form cyclic dimeric esters (formed by the esterification of two molecules of the acid) called lactides, whereas the 3-…. Read More. Bronsted Lowry Acid and Base - Chemistry Video | Clutch Prep Transcript. Hey guys, in this new video, we're going to put to practice some of the concepts we learned about Bronsted-Lowry acids and bases. Let's take a look at the first example. Here it says: Identify the acid, the base, the conjugate acid and the conjugate base based in the following reactions. Here we have HF aqueous plus H2O aqueous ...
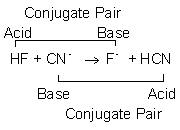
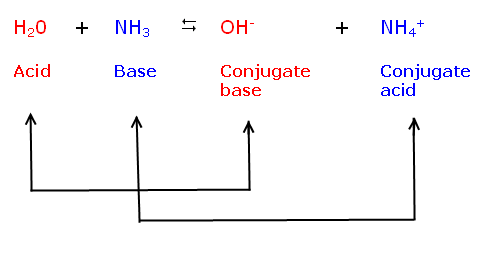
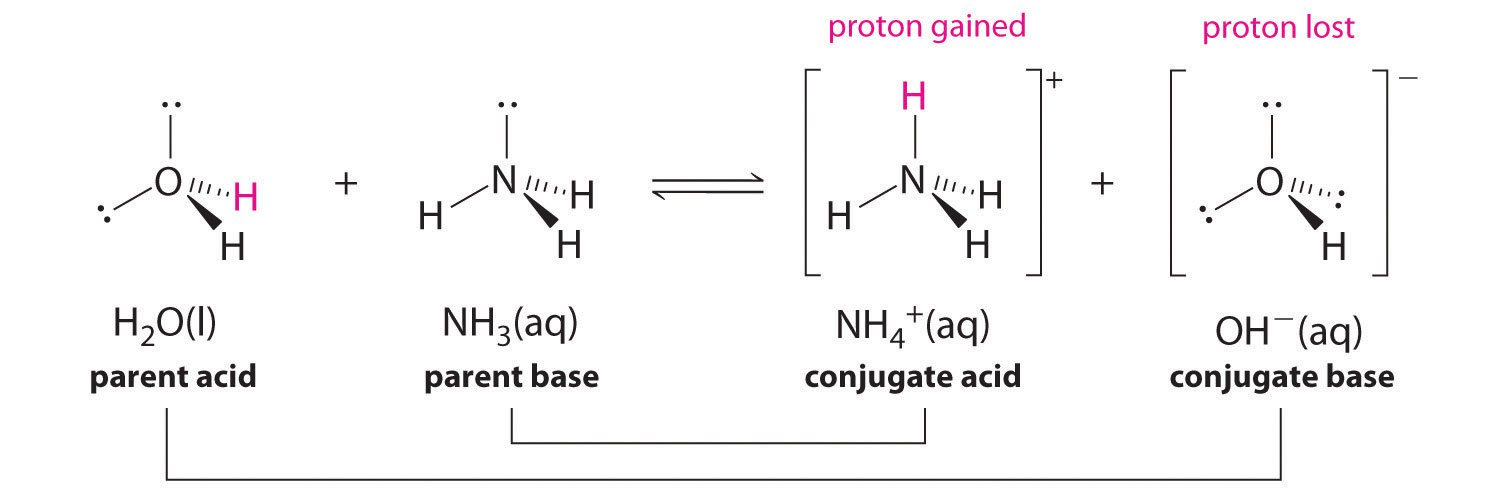
7.1: Acids and Bases - Chemistry LibreTexts This definition is not wrong; it is simply limited. Acids may be compounds such as HCl or H 2 SO 4, organic acids like acetic acid () or ascorbic acid (vitamin C), or H 2 O. Anions (such as , , , and ) and cations (such as , , and ) may also act as acids. Bases fall into the same three categories. Bases may be neutral molecules (such as , , and ... Chem 106 - Sapling Questions - Topic 3 Flashcards | Quizlet Label each reactant and product in this reaction as a Brønsted acid or base. H2Y- + H2Z- <-> H3Y + H3-2 Conjugate pairs H2Y- base-acid H3Y H2Z- acid-base H3-2 conjugate acid of a base is formed by adding a proton (ion) to the base conjugate base of an acid is formed by removing a proton from the acid. Chem 1412 Hw 10 Flashcards | Quizlet Consider three generic acids with the following relative strengths: HX > HY > HZ Rank the strengths of their conjugate bases. Strongest Base Z- Y- X- Weakest Base 6. Complete these Bronsted-Lowry reactions. HS^- + H^+ = H2S HS^- + OH^- = HO+S^2- 7. Rank the oxoacids of Iodine according to strength. Strongest Acid HIO4 HIO3 HIO2 HIO 8. Solved Identify each reactant and product in this reaction - Chegg Identify each reactant and product in this reaction as a Brønsted acid or base. H2Y−+H2Z−↽−−⇀H3Y+HZ2−H2Y−+H2Z−↽−−⇀H3Y+HZ2− Expert Answer 100% (7 ratings) 1st step All steps Final answer Step 1/2 bronsted acid donates H + and bronsted base accepts H + View the full answer Step 2/2 Final answer Previous question Next question
Label each reactant and product in this reaction as a Bronsted acid or ... For each of the following acid-base reactions, ~rewrite the reaction using complete Lewis structures for all reactants and products ~identify the Bronsted acid, the Bronsted base, the conjugate acid Write the equation for the reaction of HSO_3^+ with water in which the ion acts as an acid. Label each reactant and product in this reaction as a Bronsted acid or ... H2Y- + H2Z- H3Y + HZ2- 1 answer below » Label each reactant and product in this reaction as a Bronsted acid or base. H2Y- + H2Z- <===> H3Y + HZ2- 1 Approved Answer GANDI J answered on June 11, 2021 5 Ratings ( 13 Votes) According to the Bronsted-Lowery acid base theory The species which donates H is called as bronstead acid. The species which... Answered: In the reaction between H2Y - and H2Z -… | bartleby In the reaction between H2Y - and H2Z - , if H2Y - acts like a base, and H2Z - acts like an acid, the products of the reaction are: A) H3Y - and HZ- B) HY- and H3Z - C) H3Y and HZ2- D) H2Y and HZ- E) H3Y and HZ Expert Solution Want to see the full answer? Check out a sample QA here See Solution star_border Inorganic Exam 2 Flashcards | Quizlet HF + CH3CH2OH -> CH3CH2OH+ + F- Identify each reactant and product in this reaction as a Brønsted acid or base. H2Y- + H2Z- -> H3Y + HZ2- Base. Acid. Acid. Base Identify the pair of species that is not a conjugate acid-base pair. H3PO4; HPO2−4 Which reactant is the Lewis acid and which is the Lewis base? FeBr3 is the Lewis Acid
6.1: What is an acid and a base? - Chemistry LibreTexts Arrhenius's definition of acids and bases. The earliest definition of acids and bases is Arrhenius's definition which states that: An acid is a substance that forms hydrogen ions H + when dissolved in water, and; A base is a substance that forms hydroxide ions OH-when dissolved in water.; or example, hydrochloric acid is an acid because it forms H + when it dissolves in water.
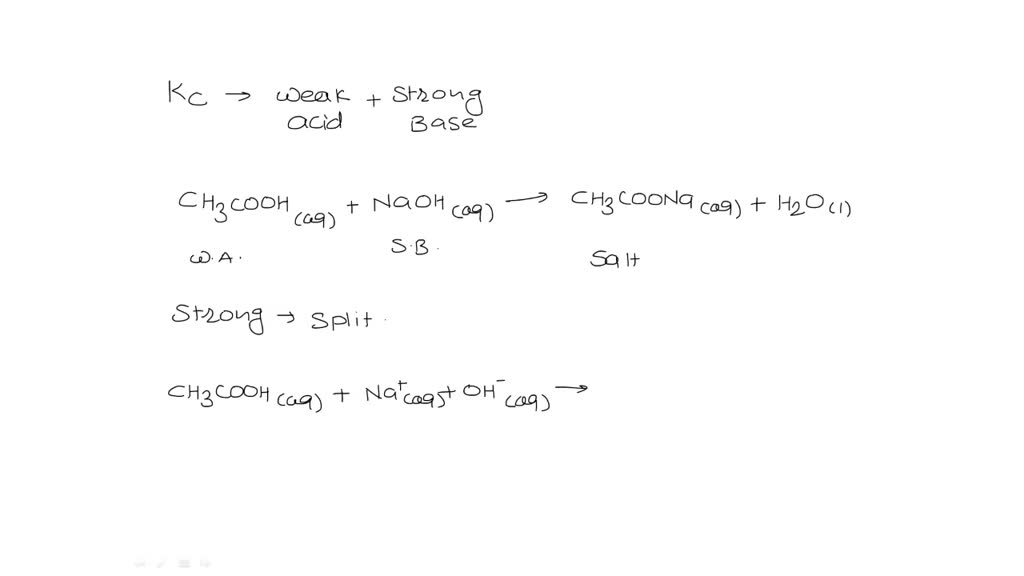
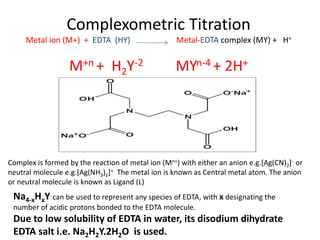
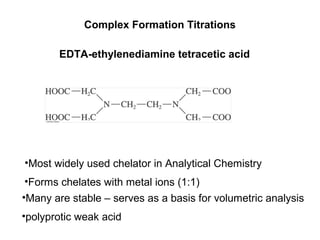
Write down the weak base (H2Y2-), hydrolysis reaction and the equilibrium constant expression. Write, the charge and mass balance equations of this aqueous solution
















![PDF] Competition between Intra and Intermolecular Triel Bonds ...](https://d3i71xaburhd42.cloudfront.net/63b055fb10130566095f8beb8a8b283d818a01a3/7-Figure4-1.png)


Post a Comment for "41 h2y acid or base"