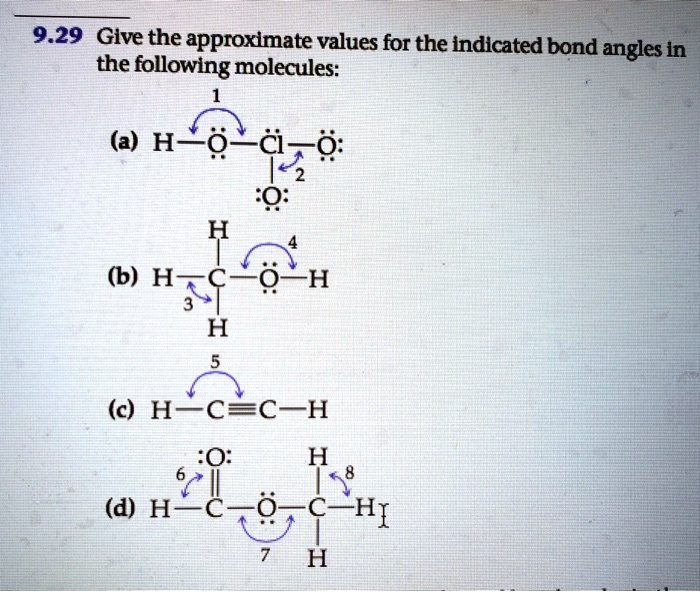
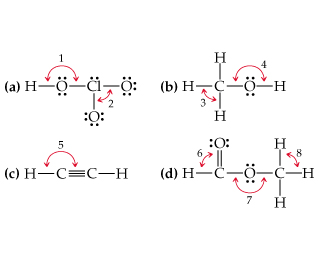
38 give the approximate values for the indicated bond angles in the following molecules
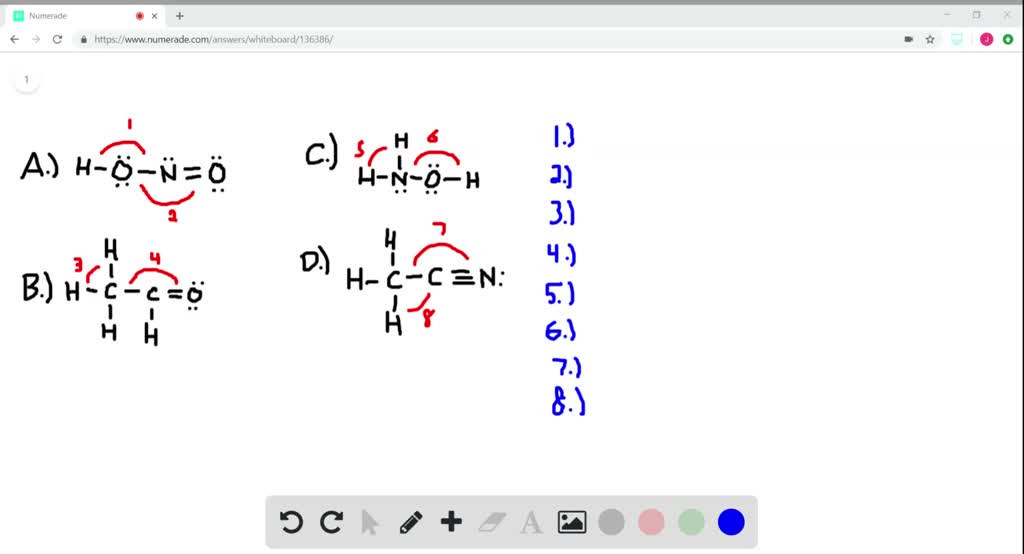
Solved 4 4. Give approximate values for the indicated bond - Chegg Give approximate values for the indicated bond angles in the following molecules. H (a) HLô N, 6 (b) H-c-cz 5 (c) H-N--- (d) H-CTCN: HH H H 1. 2. 3. 4. 5. 6. 7. 8. I 2s 2p interaction B C 12-2p interaction 0 F N Ne: 1. THEME HADIRI 11 11 11: 111 1:11 1111 11 11 11 IL 1! 11 1. 1. 1. 11 11 11 11 11 IL 11 11 1. 11 1. 3. Give approximate values for the indicated bond angles in the - SolutionInn Give approximate values for the indicated bond angles in the. Give approximate values for the indicated bond angles in the following molecules: a. b. d. 1 H- 2.
Give the approximate values for the indicated bond angles in ... - OneClass Give the approximate values for the indicated bond angles in the following molecules: Answer. + 20. Watch.

Give the approximate values for the indicated bond angles in the following molecules
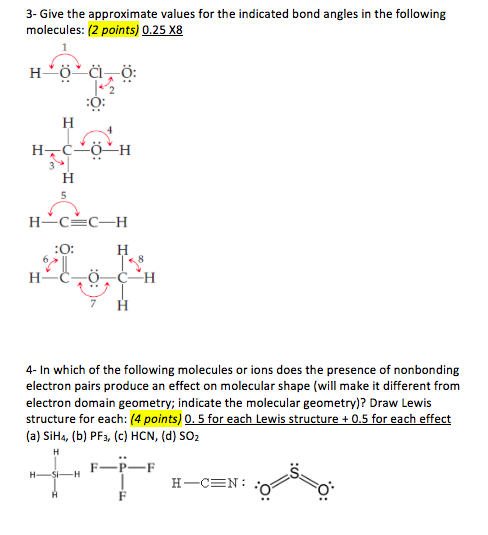
Solved Give the approximate values for the indicated bond Give the approximate values for the indicated bond angles in molecule (a): Express your answer using four significant figures. PDF Visualizing Concepts 386 CHAPTER 9 Molecular Geometry and Bonding Theories 9.18 Would you expect the non bonding electron-pair domain in NH3 to be greater or less in size than the corresponding one ---i- n PH3? 9.19 In which of these molecules or ions does the presence of nonbonding electron pairs produce an effect on molecular shape? (a) SiH4, (b) PF3, (c) J-!Br, (d) HCN, (e) S02. Answered: :O: H-N-C=C-H H H | bartleby Q: Give the approximate values for the indicated bond angles inthe following molecules: A: (a) Given compound is, In bond angle 1, oxygen atom has two bond pairs and two lone pairs (four… Q: LIAIH4 (©) CH;CH;CH;I CH;CH,CH3 + - (d) H Ph H Ph C=C H;C-C-C-H H;C ОН ОН
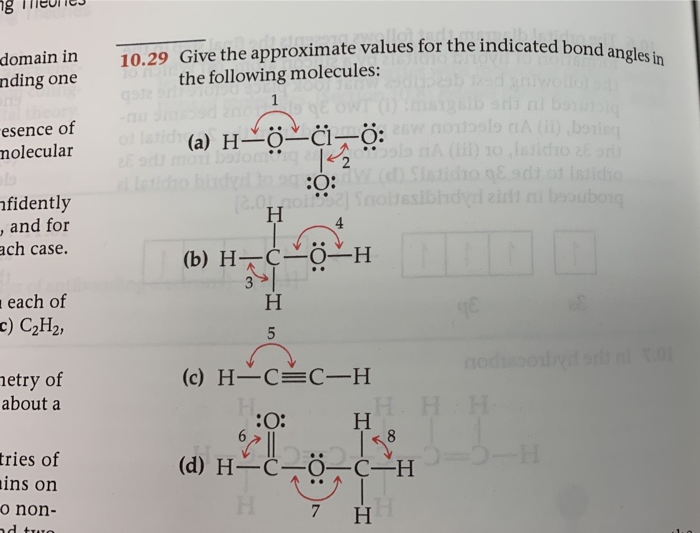
Give the approximate values for the indicated bond angles in the following molecules. PDF Chem. 1C Midterm 1 - UC Santa Barbara Complete the Lewis structures for these molecules, showing all lone pairs. Give approximate values for bond angles a through f, and give the hybridization of all carbon atoms. In acrylonitrile and methyl methacrylate indicate which atoms in each molecule must lie in the same plane. How σ bonds and how many π bonds 9.32 Give approximate values for the indicated bond angles in the ... Hicated bond angles in the 10.30 Give approximate values for the indicated bond angle following molecules: wody 1 is virbrid) brod ...of rol lo (a) Hºo-N=O zvode wolad gay wood to din 2 nodisoby is ni brod s malo bomo z oo Swed) To (b) H-C-c=... Chapter 9 Homework.docx - Give the approximate values for the indicated ... Atom, Electron, Molecule, Hund, molecular orbital configuration. Report. Text Preview: Give the approximate values for the indicated bond angles in the following molecules. What sketches are illustrating the overlap between the following orbitals on two atoms: Molecular orbital (MO) theory is based in quantum mechanics and treats the orbitals found in a molecule in a manner similar to atomic orbitals in an atom. Ch 9 Problems answers - General Chemistry assignment 9 Give approximate values for the indicated bond angles in the following molecules: 1, less than 109; 2, less than 120o 3, close to 109; 4, slightly greater than 120o 5, less than 109; 6, less than 109 7, 180 o; 8, close to 109. 9 In which of the following AF n molecules or ions is there more than one F—A—F bond angle: SiF 4 , PF 5 , SF 4 , AsF 3?
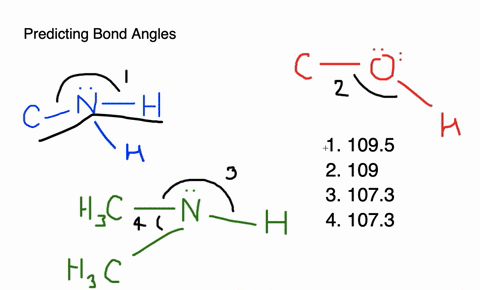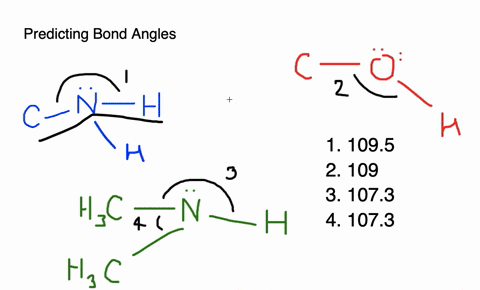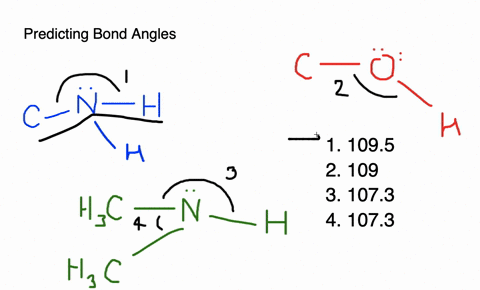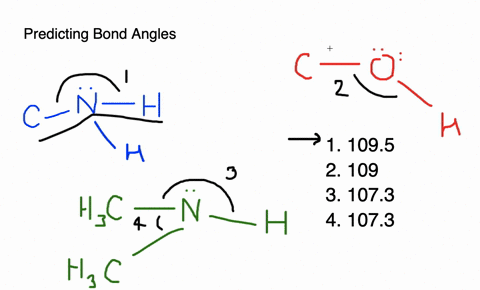
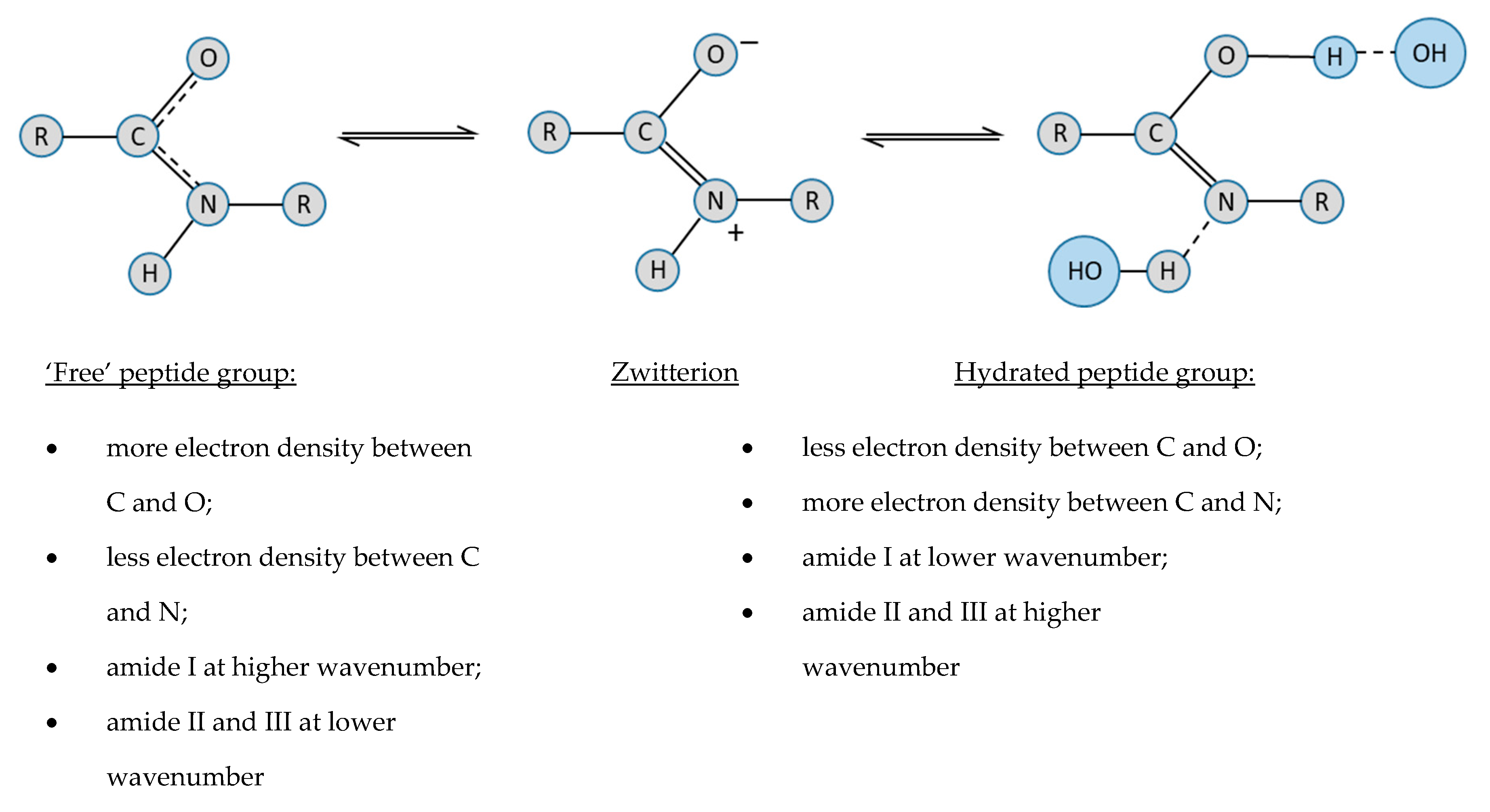
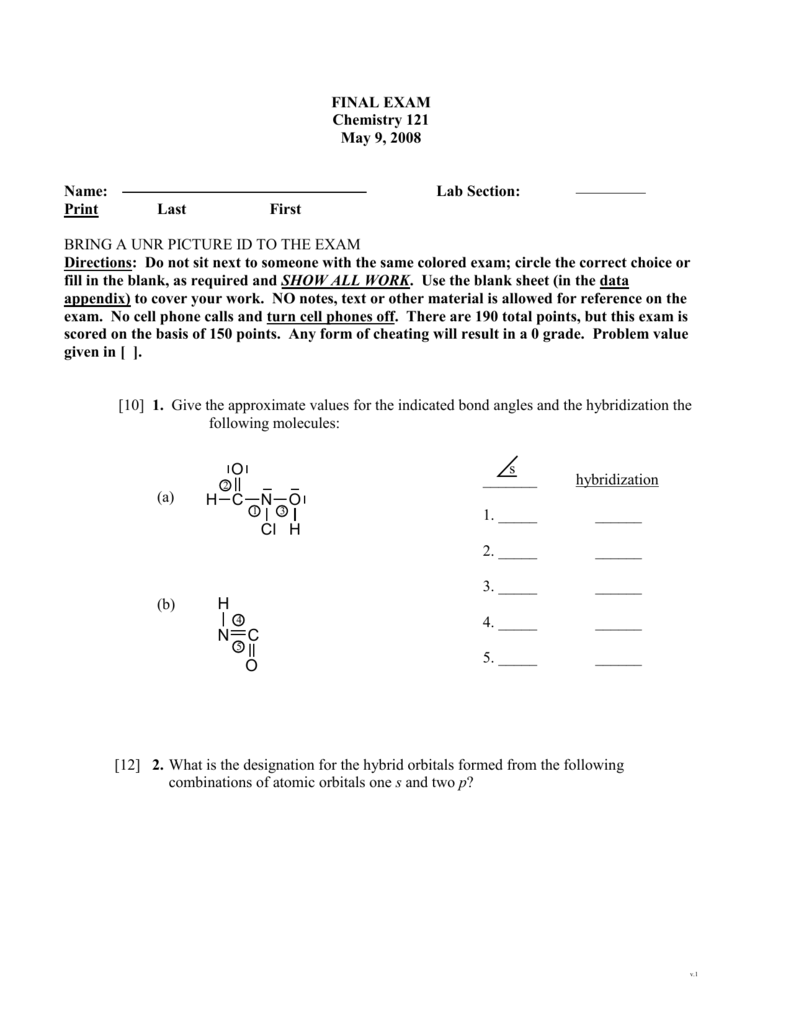
Give approximate values for the indicated bond angles in the following ... And so what we have is some bonding angles. Got one here And one here. So here we have 109.5 and here we have 120. Next, what we have is the following structure carbon single bond, carbon double bond, oxygen, hydrogen, hydrogen, hydrogen and hydrogen. So our angle in here is 109.5. We have an sp three hybridized carbon here it is 120 because we have an sp two hybridized carbon. Give the approximate values for the indicated bond angles in - SolutionInn Answer to Give the approximate values for the indicated bond angles in the following molecules:(a) (b) (c) (d) | SolutionInn Inorganic Chemistry 4th edition, Catherine Housecroft Enter the email address you signed up with and we'll email you a reset link. Give approximate values for the indicated bond angles in the following ... The molecule HCONH2 has the following approximate bond angles: Bond Angle The C-N bond length is 138 pm (contrast with C-N: 147 pm; C-N: 128 H-C-O 123º; pm; CEN: 116 pm) H-C-N N-C-0 124° Two Lewis structures can be drawn for this molecule, with the true structure being a resonance hybrid of the two.
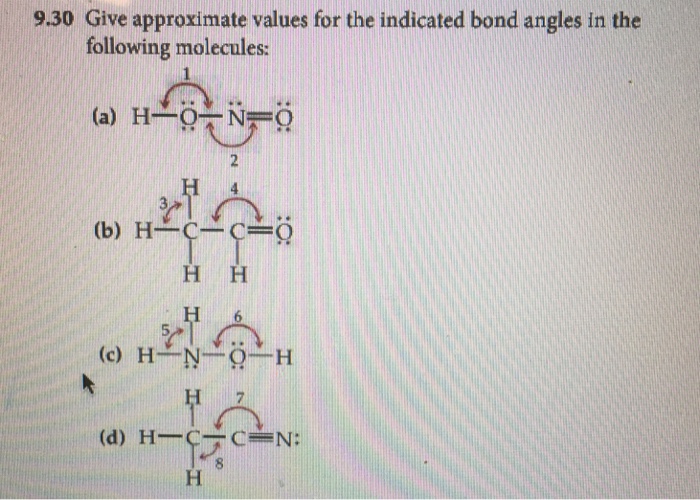
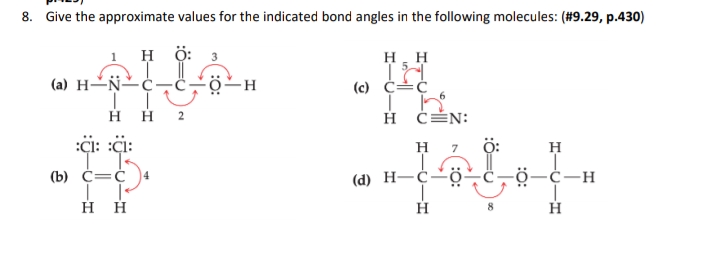
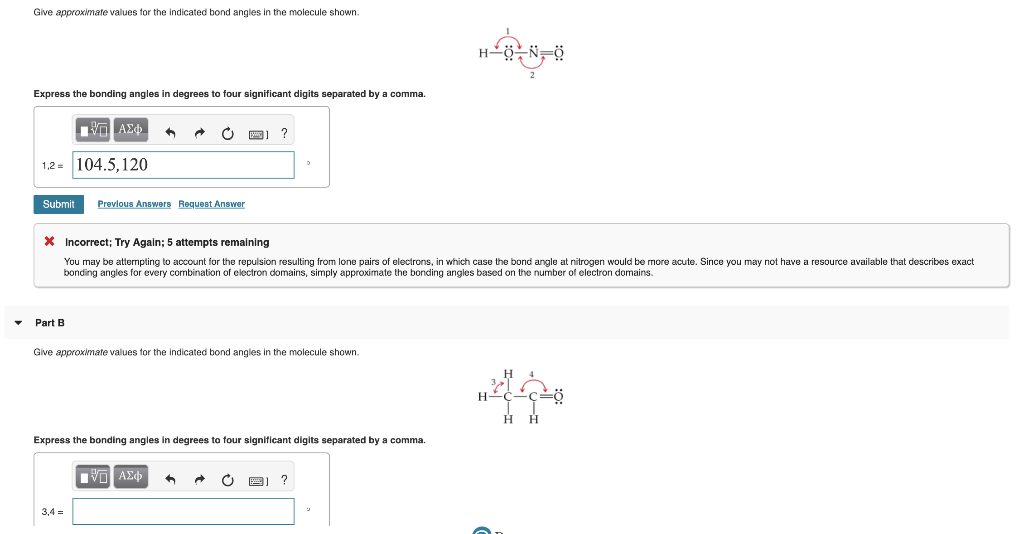
Give approximate values for the indicated bond angles in the following ... Give approximate values for the indicated bond angles in the following molecules: + 20. Watch. For unlimited access to Homework Help, a Homework+ subscription is required. Solved Give approximate values tor the indicated bond angles Express the bonding angles in degrees to four significant digits separated by a comma. Give approximate values for the indicated bond angles in the molecule ... Solved 9.30 Give the approximate values for the indicated Question: 9.30 Give the approximate values for the indicated bond angles in the following molecules: Η (a) N=N. Η Η 3 Η (b) CECCC Η Η Η Η Η Ο: Η (c) :NECC-H T Η ... Give the approximate values for the indicated bond angles ... Question: Give the approximate values for the indicated bond angles in the following molecules: 4 5 6 7 H. This problem has been solved! See the answer ...
Give approximate values for the indicated bond angles ... Question: Give approximate values for the indicated bond angles in the following molecules: This problem has been solved! See the answer ...
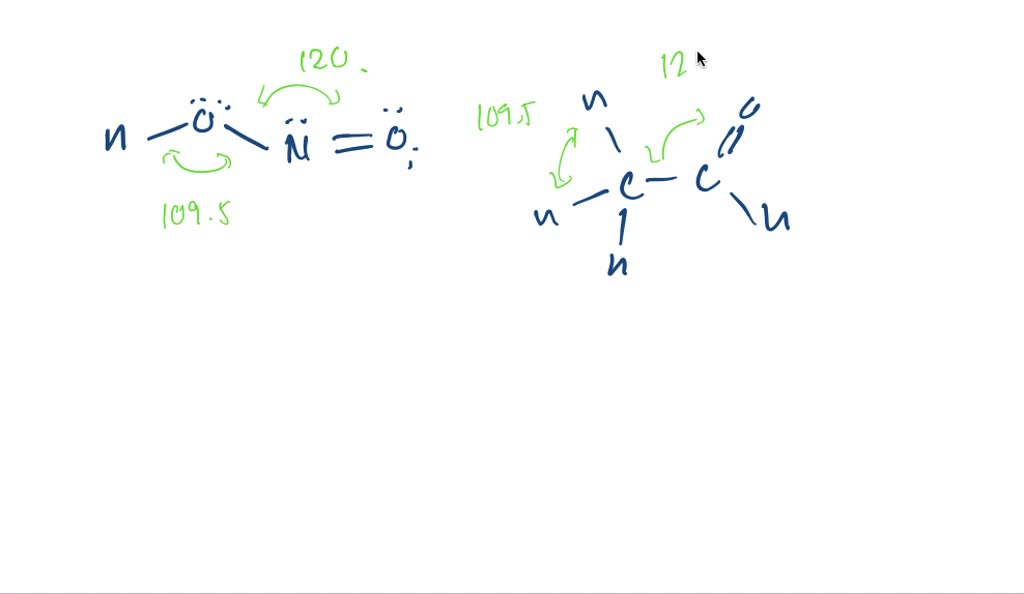
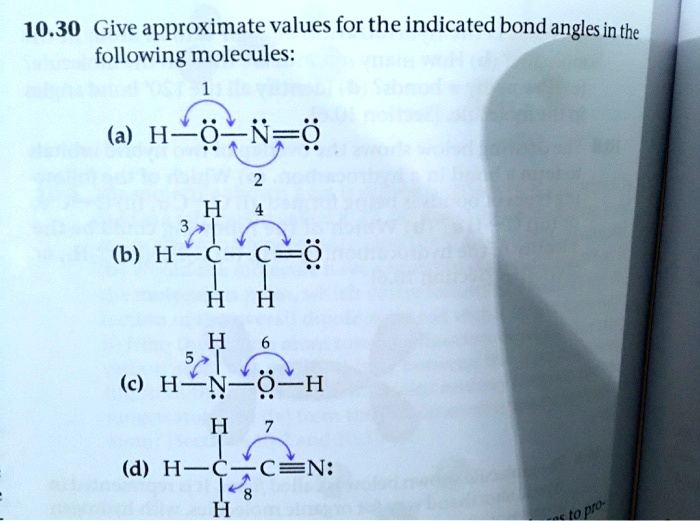
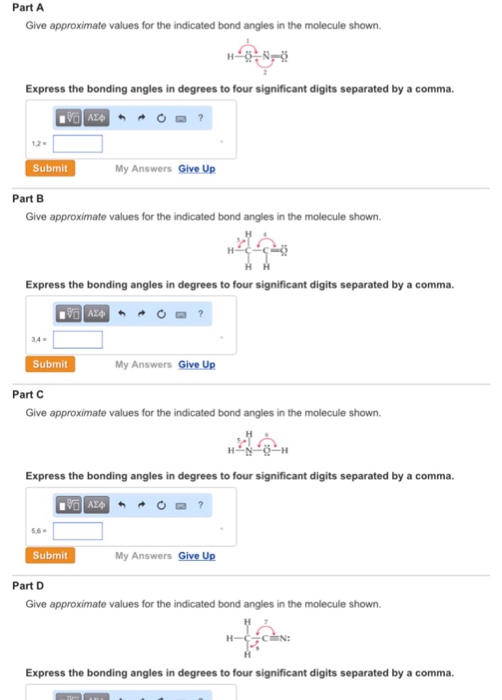
PDF Key equations - chem 1411 9.30 Give approximate values for the indicated bond angles in the following molecules: H N 1 2 4 3 O O H H H H C C O 6 5 H H N O H 7 8 H H H C C N (a) (b) (c) (d) 9.31 The three species NH2-, NH3, and NH4 + have H¬N¬H bond angles of 105°, 107°, and 109°, respectively. Explain this variation in bond angles. 9.32 In which of the following AFn molecules or ions is there more than one F¬A¬F bond angle: SiF4, PF5, SF4, AsF3?
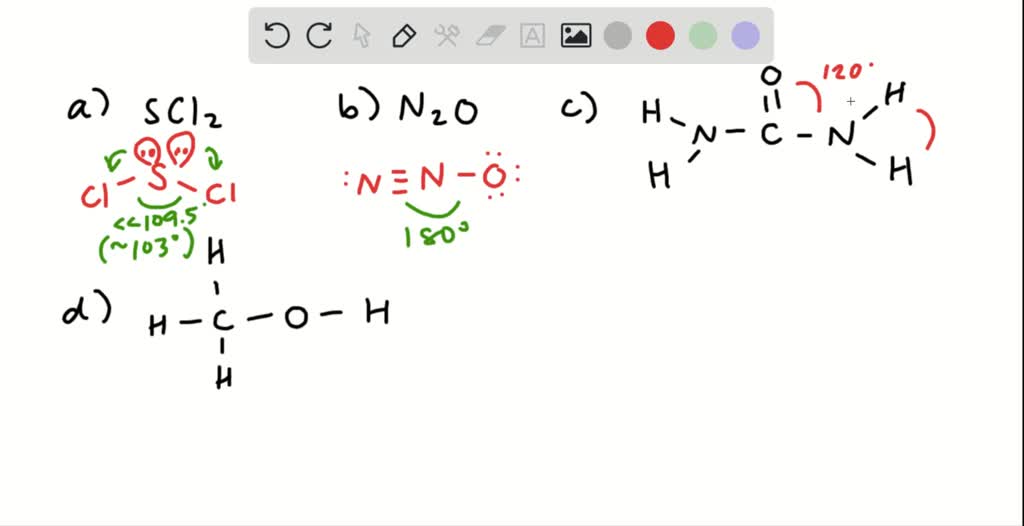
CHEM 107 CHAPTER 7 Test #3 Flashcards | Quizlet Draw a lewis structure for each of the following molecules or ions a) CS2 b) BF4-c) HNO2 (bonding is in order HONO) ... Give approximate values for the indicated bond angles a) Cl-S-Cl in SCl2 b) N-N-O in N2O c) Bond angles 1,2, and 3 in vinyl alcohol. a) 109 degrees b) 180 degrees c) #1: 120 degrees #2: 120 degrees
give approximate values for the indicated bond angles the figures given are not the actual molecular
Essay Fountain - Custom Essay Writing Service - 24/7 ... Whenever students face academic hardships, they tend to run to online essay help companies. If this is also happening to you, you can message us at course help online. We will ensure we give you a high quality content that will give you a good grade. We can handle your term paper, dissertation, a research proposal, or an essay on any topic.
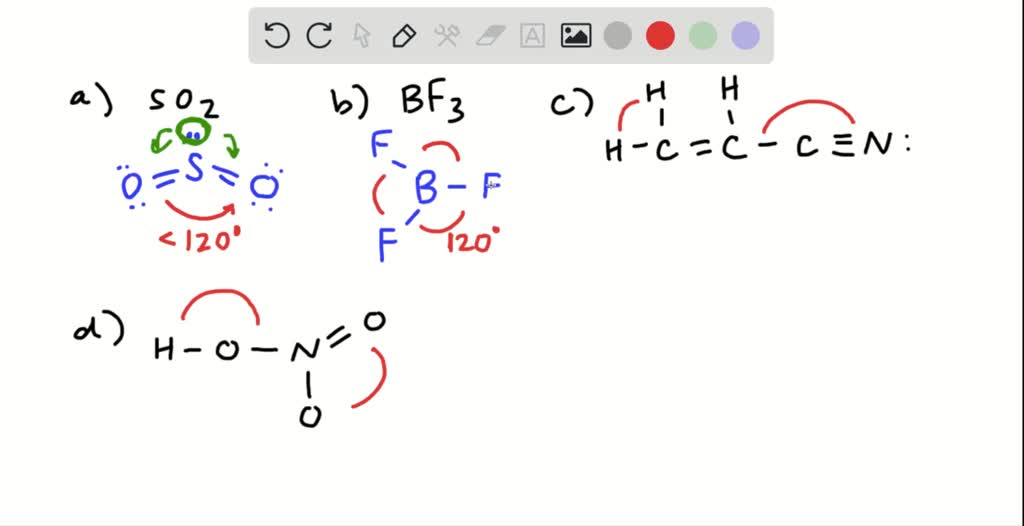
PDF CH 222 Practice Problem Set #1 - MhChem 8. Give approximate values for the indicated bond angles. a. O-S-O in SO 2 b. F-B-F angle in BF 3 c. Cl-C-Cl angle in Cl 2CO 9. Determine the formal charge on each atom in the following molecules and ions. a. NO 2+1 b. NO 21-c. NF 3 d. HNO 3 10. For each of the bonds below, Tell which atom is the more negatively charged using values of
Question : Give the approximate values for the indicated bond angles in ... This problem has been solved! Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: Give the approximate values for the indicated bond angles in the following molecules: (8 points) 4.
Bond Angles - Chemistry Video | Clutch Prep Practice: Determine the bond angle for the thiocyanate ion, SCN -. Practice: In the PCl 3 F 2 molecule the chlorine atoms exist in the equatorial positions and the fluorine atoms exist in the axial positions. Based on this information, predict the Cl-P-Cl bond angle. Practice: Determine the O-N-O bond angle for N 2 O 4, which ...
Answered: 3. Give the approximate values for the… | bartleby Give the approximate values for the indicated bond angles in the following molecules: (#9.29, p.430) 1 H ö: H. H (a) H-N-C (c) C=¢ H H 2 H ČÉN: :çi: :çi: H 7 ö: H (b) c=¢ (d) H-ċ~ö- ö-c-H нн 8.
CCG SVL Exchange Introduction: The Bio-MOE package contains a series of custom SVL applications that are used for biologics modeling. These applications, accessible from the Sequence Editor and the Database Viewer menus, include tools for antibody humanization and back mutation prioritization, liability detection, patch visualization, and PTM motif annotation, aid importing and exporting collections of ...
Give approximate values for the indicated bond angles in the molecule ... See full lesson here: I. from Manha...
Extended tight‐binding quantum chemistry methods In total, 941 bond lengths and 2,846 bond angles are compared. GFN1- and GFN2-xTB both predict distinctive bond angles around the metal atom as well as metal–ligand bond lengths with good accuracy (bond angles/bond lengths: MAD = 4.0°/7.5 p.m. and 3.9°/8.3 p.m. for GFN1- and GFN2-xTB, respectively; cf. Figure 7b 110 PM6-D3H4, 110, 111 and ...
Give approximate values for the indicated bond angles in the following ... The molecule HCONH2 has the following approximate bond angles: Bond Angle The C-N bond length is 138 pm (contrast with C-N: 147 pm; C-N: 128 H-C-O 123º; pm; CEN: 116 pm) H-C-N N-C-0 124° Two Lewis structures can be drawn for this molecule, with the true structure being a resonance hybrid of the two.
How do I determine the bond angle in a molecule? | Socratic 3. Use the VSEPR shape to determine the angles between the electron domains. From elementary math, we know that a circle is composed of 360 °. We divide this number by the number of electron domains and get (360 °)/3= 120 °. Thus, the bond angles in "BF"_3 are 120 °. This website will be a useful help in understanding how the above method works.
Give approximate values for the indicated bond angles ... Question: Give approximate values for the indicated bond angles in the molecule shown. Express the bonding angles in degrees to four significant digits ...
(Get Answer) - Give approximate values for the indicated bond angles ... Give approximate values for the indicated bond angles in themolecules shown: Express your answer using four significant figures. Enter youranswers numerically ...
Solved Give approximate values for the indicated bond angles Question: Give approximate values for the indicated bond angles in the following molecules. This problem has been solved! See the answer ...
Answered: Give approximate values for the… | bartleby Transcribed Image Text: Give approximate values for the indicated bond angles. a. H-S-H in H,S O 90° O 109.5° 120° O 180° b. I-C-N in ICN 90° O 109.5° 120° O 180° c. the following bonds in vinyl alcohol нн H-c=c-ö-H Н-С-Н 90° O 109.5° O 120° O 180° C-C-O 90° O 109.5° 120° O 180° C-O-H 90° O 109.5° O 120° O 180°.
Engineering Chemistry by Jain & Jain - Academia.edu Enter the email address you signed up with and we'll email you a reset link.
Bond Angles and the Shapes of Molecules - University of Illinois Urbana ... Figure 7.7 illustrates these structures. Note that the angles are not exactly 120° but are remarkably close to that predicted value. Although the electron clouds of these molecules give a trigonal planar shape around each carbon atom, one describes the geometry of a molecule only on the basis of the relationships between its atoms.
PDF Problem Set #1 - MhChem 7. Draw a Lewis structure of each of the following molecules or ions. Describe the electron-pair geometry and the molecular geometry around the central atom. a. SiF 62-b. PF 5 c. SF 4 d. XeF 4 8. Give approximate values for the indicated bond angles. a. Cl-S-Cl in SCl 2 b. N-N-O in N 2O c. Bond angles 1 - 5 in phenylalanine (right), one of the ...
Molecular Mechanics Methods | Gaussian.com Jan 05, 2017 · Last updated on: 05 January 2017. [G16 Rev. C.01] Quick Links. Basis Sets; Density Functional (DFT) Methods; Solvents List SCRF
Part d give approximate values for the indicated bond - Course Hero Part D Giveapproximate values for the indicated bond angles in the molecule shown. Express the bonding angles in degrees to four significant digits separated by a comma. ANSWER: Correct The bonding angles for 7 and 8 can be approximated as 180.0 and 109.5 because they have linear and tetrahedral geometries, respectively.
Give the approximate values for the indicated bond ... - Chegg Question: Give the approximate values for the indicated bond angles in the following molecule: 2 :O: 109.5° and 109.5° 180° and 90° 90° and 90° 180° and 120° ...
Give approximate values for the indicated bond angles in the following ... Number thirty of Chapter nine. They wants to give the apartment bond partner values for the indicated bond angles of these molecules I've drawn here, Tio. Let's serve one and, ah, we're looking at the oxygen auctions has two bombs and two lone pairs, so that's going to be a bench structure. So that's going to be less than one hundred and nine ...
Answered: :O: H-N-C=C-H H H | bartleby Q: Give the approximate values for the indicated bond angles inthe following molecules: A: (a) Given compound is, In bond angle 1, oxygen atom has two bond pairs and two lone pairs (four… Q: LIAIH4 (©) CH;CH;CH;I CH;CH,CH3 + - (d) H Ph H Ph C=C H;C-C-C-H H;C ОН ОН
PDF Visualizing Concepts 386 CHAPTER 9 Molecular Geometry and Bonding Theories 9.18 Would you expect the non bonding electron-pair domain in NH3 to be greater or less in size than the corresponding one ---i- n PH3? 9.19 In which of these molecules or ions does the presence of nonbonding electron pairs produce an effect on molecular shape? (a) SiH4, (b) PF3, (c) J-!Br, (d) HCN, (e) S02.
Solved Give the approximate values for the indicated bond Give the approximate values for the indicated bond angles in molecule (a): Express your answer using four significant figures.















Post a Comment for "38 give the approximate values for the indicated bond angles in the following molecules"